The lycophytes (Subphylum Lycopodiophytina, Phylum Tracheophyta) are a clade that comprises the following three orders:
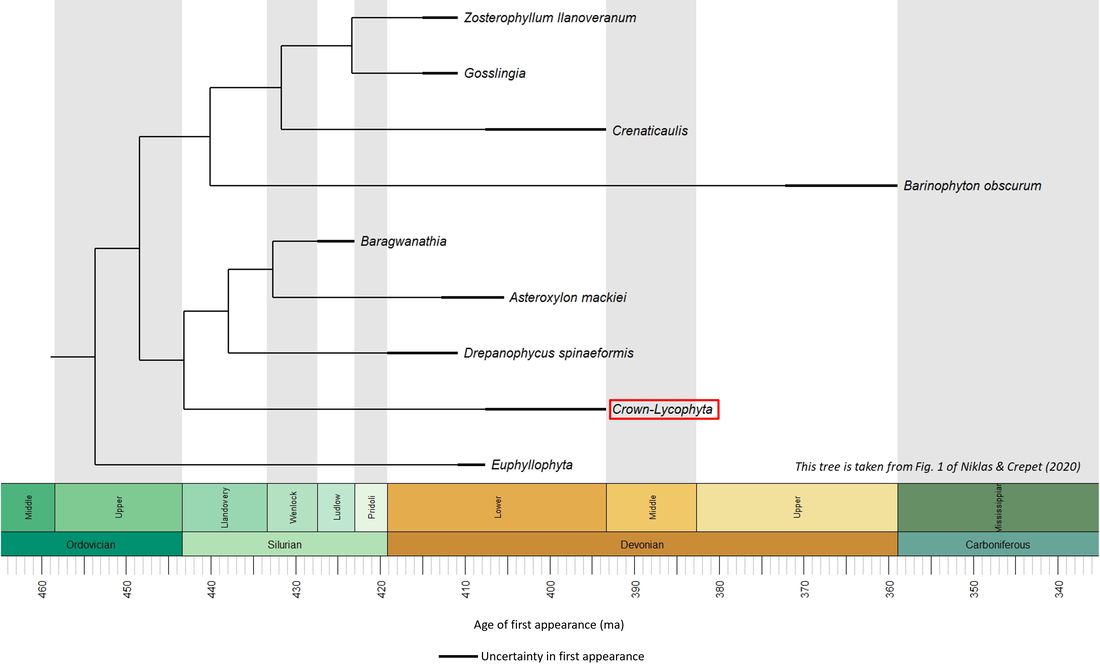
Several phylogenetic trees of the lycophyte stem group have been published recently (e.g. Cascales‐Miñana et al, 2017; Cascales‐Miñana et al, 2019; Niklas and Crepet, 2020), but the most recent of the three may be considered representative and is summarized in the following time tree:
- Isoetales (Quillworts)
- Selaginellales (Spike mosses)
- Lycopodiales (Club mosses)
Several phylogenetic trees of the lycophyte stem group have been published recently (e.g. Cascales‐Miñana et al, 2017; Cascales‐Miñana et al, 2019; Niklas and Crepet, 2020), but the most recent of the three may be considered representative and is summarized in the following time tree:
Figure 1. Time tree of the stem-Lycophyta
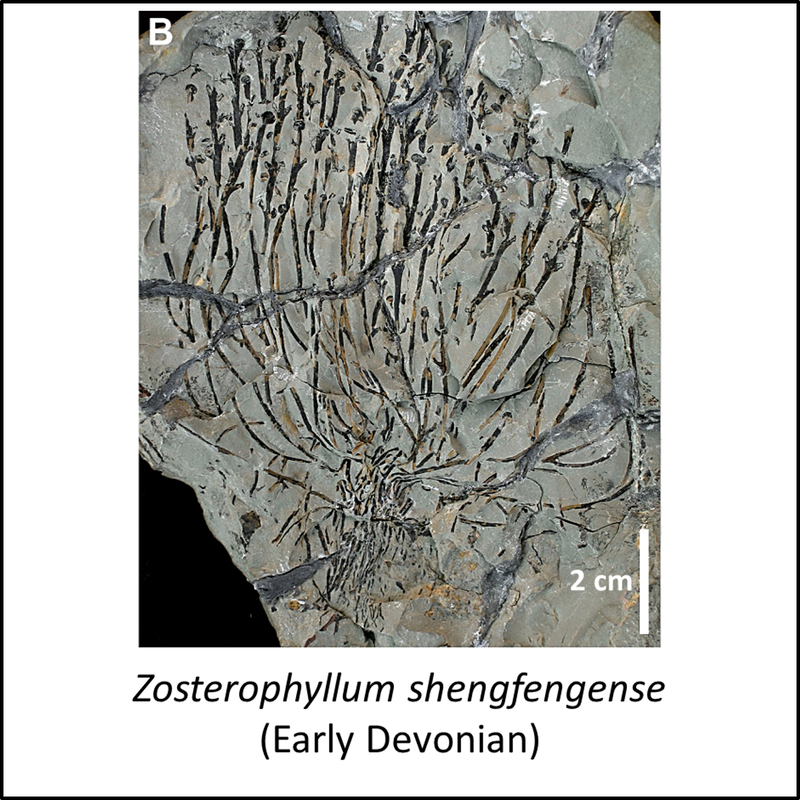
The oldest known member of the lycophyte stem group is Baragwanathia longifolia, described from the mid-Silurian (Wenlock) Jordan River Beds exposed along the Thomson River in Victoria, Australia (Lang and Cookson, 1935; Rickards, 2000; Niklas and Crepet, 2020). Another species of this genus, together with other members of the stem group for which images are available in the public domain, are shown below (click on image for a larger view):
Names in red indicate that the fossil is younger than the oldest known crown-group fossil.
Figure 2. Images of stem-Lycophyta
The above images are ordered from most basal to most crownward, but there are too few of them to allow evolutionary trends to be suggested. In any case, microphylls are clearly visible in Barinophyton, Drepanophycus and Baragwanathia, with sporangia also evident in Gosslingia and Baragwanathia.
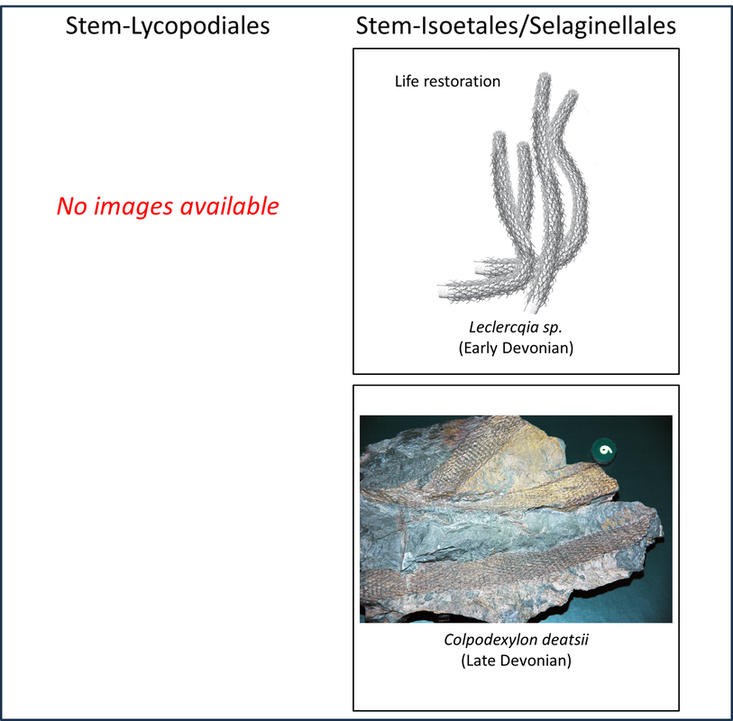
Some idea of the nature of the transition from the stem group to the crown group of the lycophytes can be obtained from a comparison of the above images with the examples of crown-Lycophyta shown below:
Some idea of the nature of the transition from the stem group to the crown group of the lycophytes can be obtained from a comparison of the above images with the examples of crown-Lycophyta shown below:
Figure 3. Examples of crown-Lycophyta
The above time tree (Figure 1) indicates that the lycophyte stem group developed from mid-Silurian to Early Devonian time, representing a stem-to-crown transition of at least 15 million years.
References
Cascales‐Miñana, B., & Gerrienne, P. (2017). Teruelia diezii gen. et sp. nov.: an early polysporangiophyte from the Lower Devonian of the Iberian Peninsula. Palaeontology, 60(2), 199-212.
Cascales-Miñana, B., Steemans, P., Servais, T., Lepot, K., & Gerrienne, P. (2019). An alternative model for the earliest evolution of vascular plants. Lethaia, 52(4), 445-453.
De Queiroz, K., Cantino, P. D., & Gauthier, J. A. (Eds.). (2020). Phylonyms: a Companion to the PhyloCode. CRC Press.
Lang, W. H., & Cookson, I. C. (1935). IX-On a flora, including vascular land plants, associated with Monograptus, in rocks of Silurian age, from Victoria, Australia. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 224(517), 421-449.
Niklas, K. J., & Crepet, W. L. (2020). Morphological (and not anatomical or reproductive) features define early vascular plant phylogenetic relationships. American Journal of Botany, 107(3), 477-488.
Rickards, R. B. (2000). The age of the earliest club mosses: the Silurian Baragwanathia flora in Victoria, Australia. Geological Magazine, 137(2), 207-209.
Taylor, E. L., Taylor, T. N., & Krings, M., Eds. (2009). Paleobotany: the biology and evolution of fossil plants (Second Edition). Academic Press.
Tomescu, A. M. (2009). Megaphylls, microphylls and the evolution of leaf development. Trends in plant science, 14(1), 5-12.
Cascales-Miñana, B., Steemans, P., Servais, T., Lepot, K., & Gerrienne, P. (2019). An alternative model for the earliest evolution of vascular plants. Lethaia, 52(4), 445-453.
De Queiroz, K., Cantino, P. D., & Gauthier, J. A. (Eds.). (2020). Phylonyms: a Companion to the PhyloCode. CRC Press.
Lang, W. H., & Cookson, I. C. (1935). IX-On a flora, including vascular land plants, associated with Monograptus, in rocks of Silurian age, from Victoria, Australia. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 224(517), 421-449.
Niklas, K. J., & Crepet, W. L. (2020). Morphological (and not anatomical or reproductive) features define early vascular plant phylogenetic relationships. American Journal of Botany, 107(3), 477-488.
Rickards, R. B. (2000). The age of the earliest club mosses: the Silurian Baragwanathia flora in Victoria, Australia. Geological Magazine, 137(2), 207-209.
Taylor, E. L., Taylor, T. N., & Krings, M., Eds. (2009). Paleobotany: the biology and evolution of fossil plants (Second Edition). Academic Press.
Tomescu, A. M. (2009). Megaphylls, microphylls and the evolution of leaf development. Trends in plant science, 14(1), 5-12.
Image credits – stem-Lycophyta
- Figure 2 (Barinophyton citrulliforme): Skye McDavid, CC BY 4.0 <https://creativecommons.org/licenses/by/4.0>, via Wikimedia Commons
- Figure 2 (Gosslingia breconensis): By permission of Hans Steur (https://steurh.home.xs4all.nl/engoudpl/egoss2.html)
- Figure 2 (Zosterophyllum llanoveranum, fossil): Ghedoghedo, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
- Figure 2 (Zosterophyllum llanoveranum, life restoration): MUSE (Museo delle Scienze, Trento, Italy), CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
- Figure 2 (Drepanophycus sp.): Ghedoghedo, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
- Figure 2 (Baragwanathia brevifolia): Open Access article Pšenička, J., Bek, J., Frýda, J., Žárský, V., Uhlířová, M., & Štorch, P. (2021). Dynamics of Silurian plants as response to climate changes. Life, 11(9), 906.
- Figure 2 (Asteroxylon sp.): Ghedoghedo, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
- Figure 2 (Asteroxylon mackiei, life restoration): Alexander J Hetherington, Siobhán L Bridson, Anna Lee Jones, Hagen Hass, Hans Kerp, Liam Dolan, CC BY 4.0 <https://creativecommons.org/licenses/by/4.0>, via Wikimedia Commons
- Figure 3 (Leclercqia sp.): Falconaumanni, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
- Figure 3 (Colpodexylon deatsii): James St. John, CC BY 2.0 <https://creativecommons.org/licenses/by/2.0>, via Wikimedia Commons