This page is the first in a series of pages that covers fossils associated with the stem group that leads to the crown group of the vascular plants (Phylum Tracheophyta, Superphylum Embryophyta). and with the stem groups of the clades within the crown group.
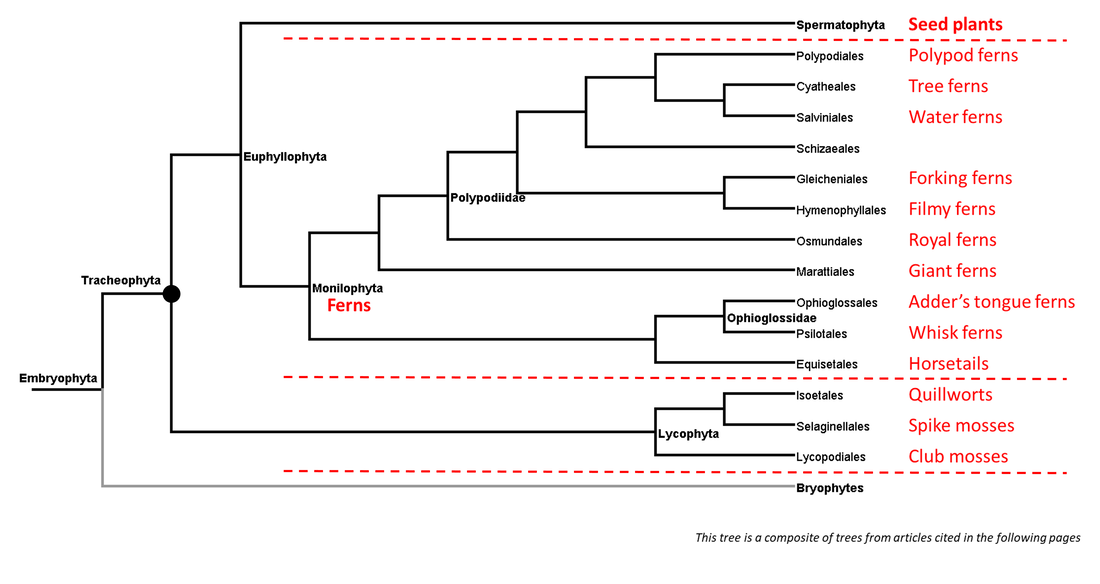
A summary of the phylogeny of the tracheophyte clade is shown below:
A summary of the phylogeny of the tracheophyte clade is shown below:
Figure 1. Summarized phylogenetic tree of the vascular plants
Each page of this website deals with a single branch of the phylogenetic tree. Links to the pages corresponding to the branches shown above (those for which adequate data are available) can be found under the Navigation tab above.
The tetrapod stem branches not represented in this website are listed in the following table. In each case the column highlighted by the letter X indicates the reason for not including that branch:
The tetrapod stem branches not represented in this website are listed in the following table. In each case the column highlighted by the letter X indicates the reason for not including that branch:
This page thus covers the stem-group tracheophytes.
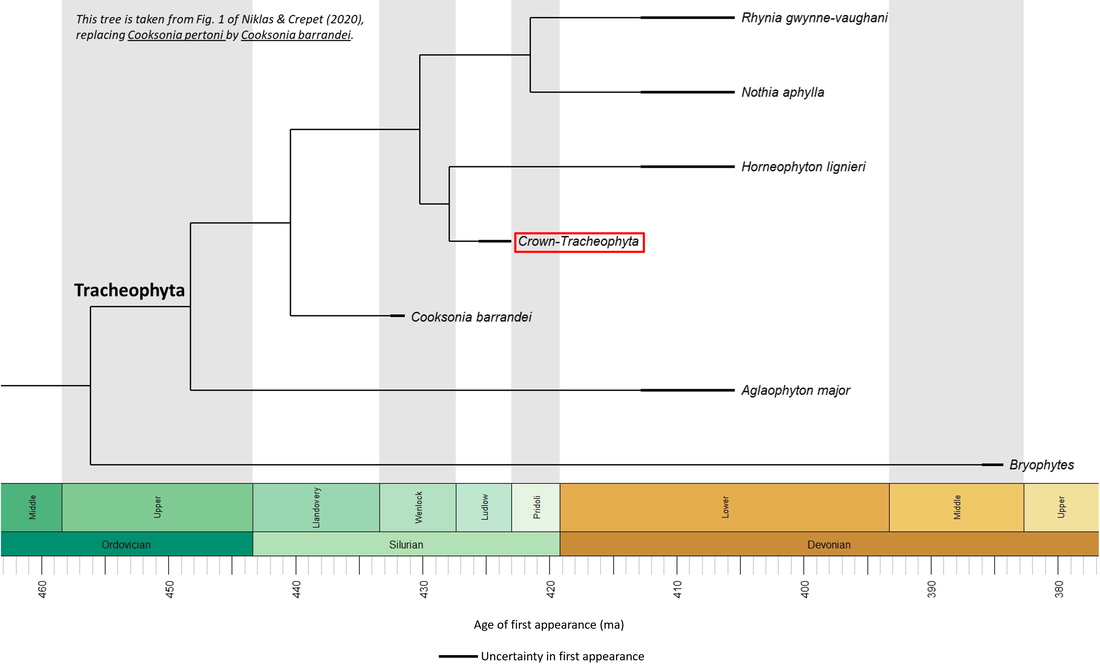
The phylogeny of the stem-Tracheophyta is still contentious. Various proposals have been made in recent years (e.g. Cascales‐Miñana and Gerrienne, 2017; Cascales-Miñana et al, 2019; Niklas and Crepet, 2020). The version of Niklas and Crepet (2020) is generally representative of the consensus developed over the last few decades (following Kenrick and Crane, 1997); it is summarized in the following time tree:
The phylogeny of the stem-Tracheophyta is still contentious. Various proposals have been made in recent years (e.g. Cascales‐Miñana and Gerrienne, 2017; Cascales-Miñana et al, 2019; Niklas and Crepet, 2020). The version of Niklas and Crepet (2020) is generally representative of the consensus developed over the last few decades (following Kenrick and Crane, 1997); it is summarized in the following time tree:
Figure 2. Time tree of the stem-Tracheophyta
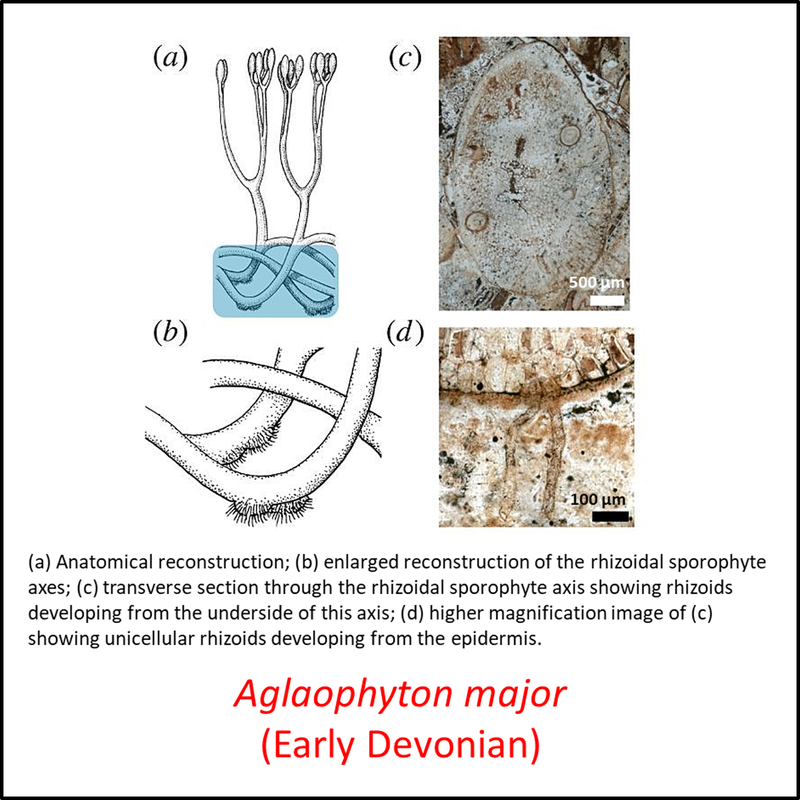
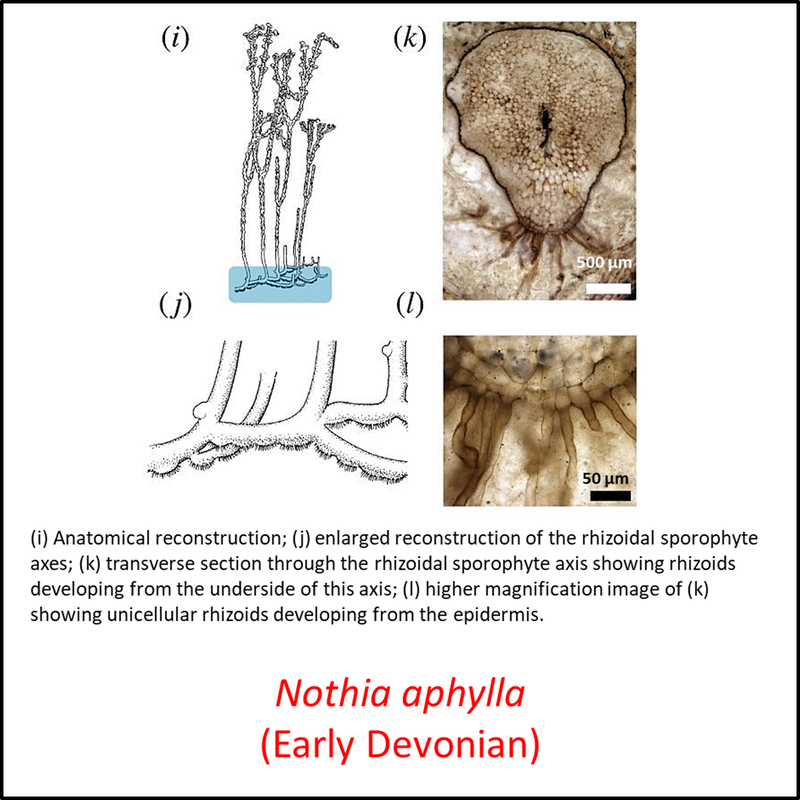
The oldest known member of the tracheophyte total group is Cooksonia barrandei (Libertín et al, 2018a), found in the Motol Formation of mid-Silurian (Wenlock) age at the Špičatý vrch - Barrandovy jámy fossil site in the Czech Republic (Libertín et al, 2018). This is assumed here to be a stem tracheophyte based on its belonging to the same genus as the generally recognized stem-group tracheophyte Cooksonia pertoni (Edwards and Kenrick, 2015). Several species of the genus Cooksonia are illustrated below, together with other members of the stem group included in the above time tree (for a larger view, click on image):
Names in red indicate that the fossil is younger than the oldest known crown-group fossil.
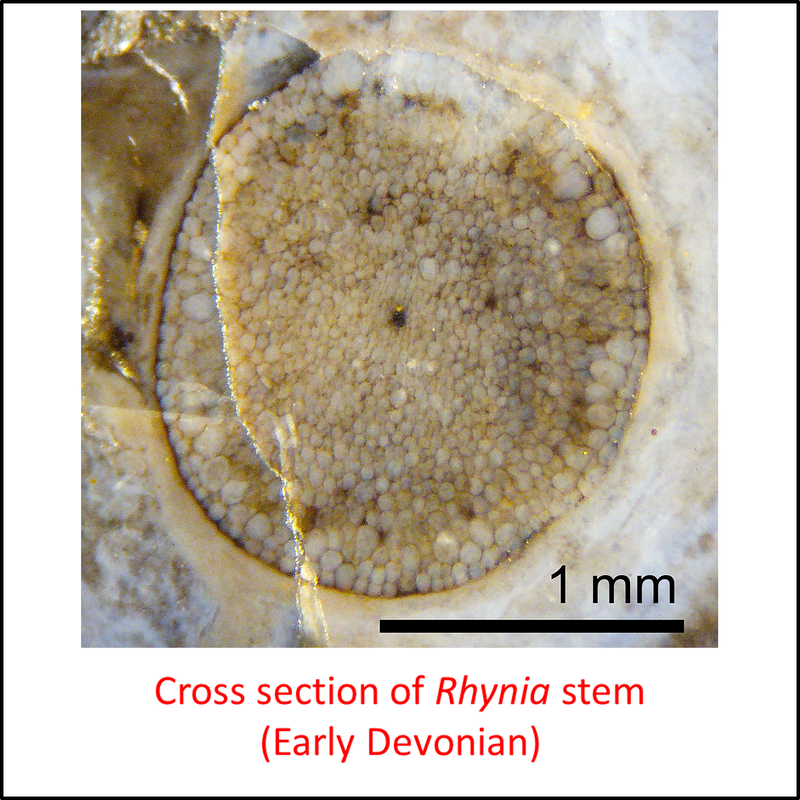
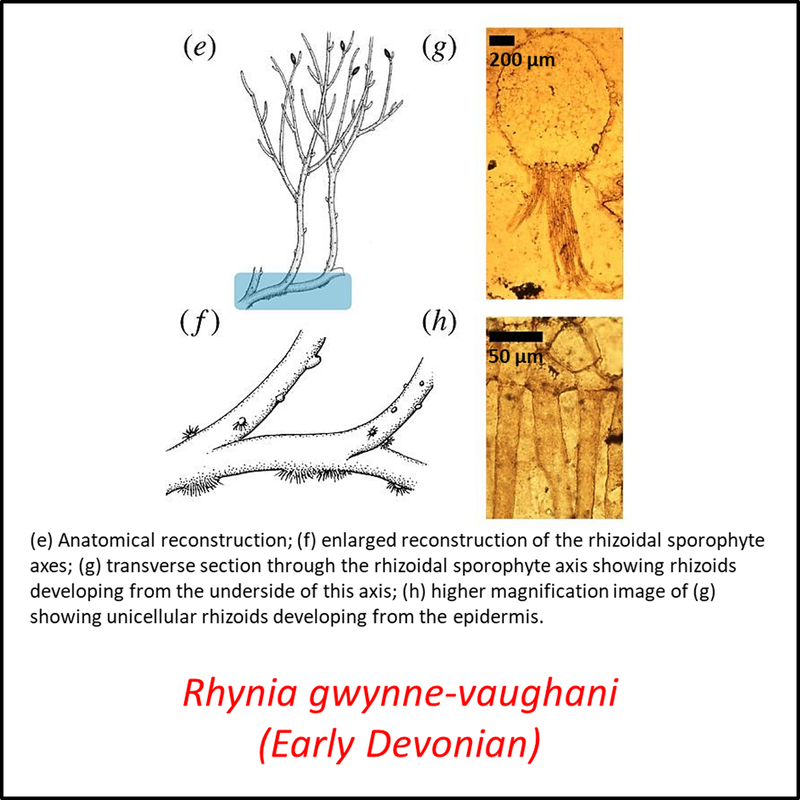
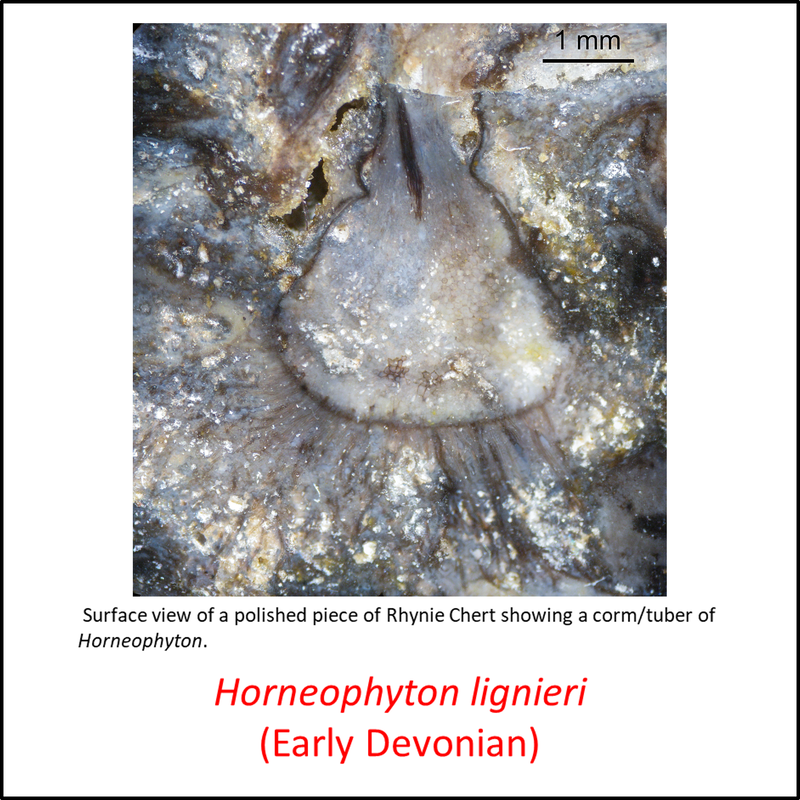
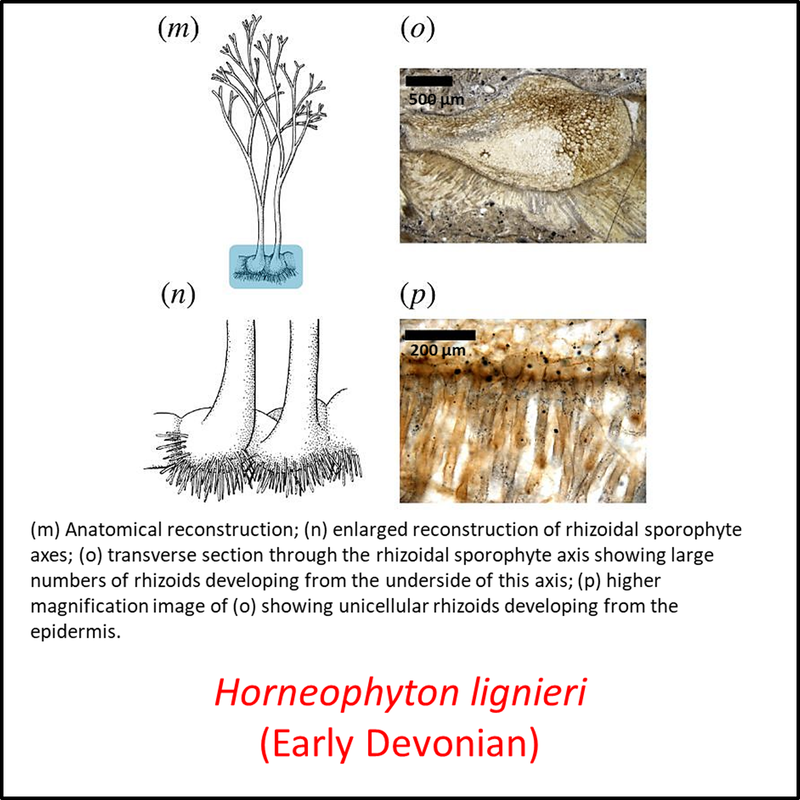
Figure 3. Images of stem-Tracheophyta
The above images are ordered from most basal to most crownward but no obvious trends can be seen.
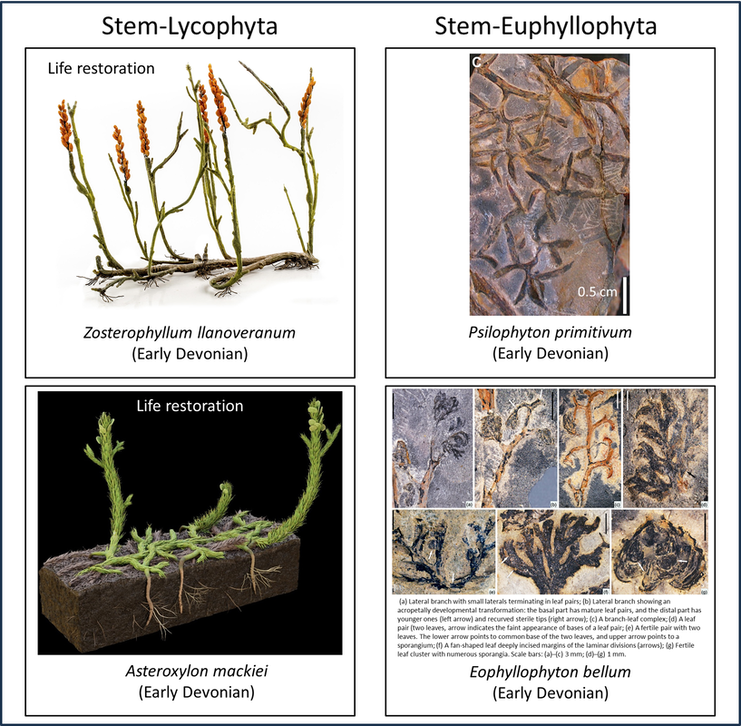
Some idea of the nature of the transition from the stem group to the crown group of the vascular plants can be derived from a comparison of the above images with the examples of crown-Tracheophyta shown below:
Some idea of the nature of the transition from the stem group to the crown group of the vascular plants can be derived from a comparison of the above images with the examples of crown-Tracheophyta shown below:
Figure 4. Examples of crown-Tracheophyta
The available fossil data (see Figure 2 above) indicate that the tracheophyte stem group developed within mid-Silurian time, representing a minimum stem-to-crown transition period of 4.0 million years.
References
Cascales‐Miñana, B., & Gerrienne, P. (2017). Teruelia diezii gen. et sp. nov.: an early polysporangiophyte from the Lower Devonian of the Iberian Peninsula. Palaeontology, 60(2), 199-212.
Cascales-Miñana, B., Steemans, P., Servais, T., Lepot, K., & Gerrienne, P. (2019). An alternative model for the earliest evolution of vascular plants. Lethaia, 52(4), 445-453.
De Queiroz, K., Cantino, P. D., & Gauthier, J. A. (Eds.). (2020). Phylonyms: a Companion to the PhyloCode. CRC Press.
Edwards, D., & Kenrick, P. (2015). The early evolution of land plants, from fossils to genomics: a commentary on Lang (1937)‘On the plant-remains from the Downtonian of England and Wales'. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1666), 20140343.
Garwood, R. J., Oliver, H., & Spencer, A. R. (2020). An introduction to the Rhynie chert. Geological Magazine, 157(1), 47-64.
Kenrick, P., & Crane, P. R. (1997). The origin and early evolution of plants on land. Nature, 389(6646), 33-39.
Kerp, H. (2018). Organs and tissues of Rhynie chert plants. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1739), 20160495.
Libertín, M., Kvaček, J., Bek, J., Žárský, V., & Štorch, P. (2018). Sporophytes of polysporangiate land plants from the early Silurian period may have been photosynthetically autonomous. Nature plants, 4(5), 269-271.
Morris, J. L., Puttick, M. N., Clark, J. W., Edwards, D., Kenrick, P., Pressel, S., ... & Donoghue, P. C. (2018). The timescale of early land plant evolution. Proceedings of the National Academy of Sciences, 115(10), E2274-E2283.
Niklas, K. J., & Crepet, W. L. (2020). Morphological (and not anatomical or reproductive) features define early vascular plant phylogenetic relationships. American Journal of Botany, 107(3), 477-488.
Cascales-Miñana, B., Steemans, P., Servais, T., Lepot, K., & Gerrienne, P. (2019). An alternative model for the earliest evolution of vascular plants. Lethaia, 52(4), 445-453.
De Queiroz, K., Cantino, P. D., & Gauthier, J. A. (Eds.). (2020). Phylonyms: a Companion to the PhyloCode. CRC Press.
Edwards, D., & Kenrick, P. (2015). The early evolution of land plants, from fossils to genomics: a commentary on Lang (1937)‘On the plant-remains from the Downtonian of England and Wales'. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1666), 20140343.
Garwood, R. J., Oliver, H., & Spencer, A. R. (2020). An introduction to the Rhynie chert. Geological Magazine, 157(1), 47-64.
Kenrick, P., & Crane, P. R. (1997). The origin and early evolution of plants on land. Nature, 389(6646), 33-39.
Kerp, H. (2018). Organs and tissues of Rhynie chert plants. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1739), 20160495.
Libertín, M., Kvaček, J., Bek, J., Žárský, V., & Štorch, P. (2018). Sporophytes of polysporangiate land plants from the early Silurian period may have been photosynthetically autonomous. Nature plants, 4(5), 269-271.
Morris, J. L., Puttick, M. N., Clark, J. W., Edwards, D., Kenrick, P., Pressel, S., ... & Donoghue, P. C. (2018). The timescale of early land plant evolution. Proceedings of the National Academy of Sciences, 115(10), E2274-E2283.
Niklas, K. J., & Crepet, W. L. (2020). Morphological (and not anatomical or reproductive) features define early vascular plant phylogenetic relationships. American Journal of Botany, 107(3), 477-488.
Image credits – stem-Tracheophtya
- Header (Woody Dicot Stem: Primary Phloem and Xylem in One Year Tilia): Berkshire Community College Bioscience Image Library, CC0, via Wikimedia Commons
- Figure 3 (Aglaophyton major): Open Access article Hetherington, A. J., & Dolan, L. (2018). Bilaterally symmetric axes with rhizoids composed the rooting structure of the common ancestor of vascular plants. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1739), 20170042.
- Figure 3 (Cooksonia barrandei): Open Access article Pšenička, J., Bek, J., Frýda, J., Žárský, V., Uhlířová, M., & Štorch, P. (2021). Dynamics of Silurian plants as response to climate changes. Life, 11(9), 906.
- Figure 3 (Cooksonia sp., fossil): Open Access article Pšenička, J., Bek, J., Frýda, J., Žárský, V., Uhlířová, M., & Štorch, P. (2021). Dynamics of Silurian plants as response to climate changes. Life, 11(9), 906.
- Figure 3 (Cooksonia sp. life restoration): MUSE, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
- Figure 3 (Cooksonia cf. hemisphaerica): Open Access article Pšenička, J., Bek, J., Frýda, J., Žárský, V., Uhlířová, M., & Štorch, P. (2021). Dynamics of Silurian plants as response to climate changes. Life, 11(9), 906.
- Figure 3 (Nothia aphylla): Open Access article Hetherington, A. J., & Dolan, L. (2018). Bilaterally symmetric axes with rhizoids composed the rooting structure of the common ancestor of vascular plants. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1739), 20170042.
- Figure 3 (Rhynia gwynne-vaughani, fossil): Peter Coxhead, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
- Figure 3 (Rhynia gwynne-vaughani, fossil and reconstructions): Open Access article Hetherington, A. J., & Dolan, L. (2018). Bilaterally symmetric axes with rhizoids composed the rooting structure of the common ancestor of vascular plants. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1739), 20170042.
- Figure 3 (Rhynia gwynne-vaughani, life restoration): MUSE, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
- Figure 3 (Horneophyton lignieri, fossil with several corms or tubers): Peter Coxhead, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons
- Figure 3 (Horneophyton lignieri, fossil with magnified view of a single corm or tuber): Peter Coxhead, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
- Figure 3 (Horneophyton lignieri, fossil and reconstructions): Open Access article Hetherington, A. J., & Dolan, L. (2018). Bilaterally symmetric axes with rhizoids composed the rooting structure of the common ancestor of vascular plants. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1739), 20170042.
- Figure 4 (Zosterophyllum llanoveranum): MUSE (Museo delle Scienze, Trento, Italy), CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
- Figure 4 (Asteroxylon mackiei): Alexander J Hetherington, Siobhán L Bridson, Anna Lee Jones, Hagen Hass, Hans Kerp, Liam Dolan, CC BY 4.0 <https://creativecommons.org/licenses/by/4.0>, via Wikimedia Commons
- Figure 4 (Psilophyton primitivum): Open Access article Capel, E., Cleal, C. J., Xue, J., Monnet, C., Servais, T., & Cascales-Miñana, B. (2022). The Silurian–Devonian terrestrial revolution: diversity patterns and sampling bias of the vascular plant macrofossil record. Earth-Science Reviews, 231, 104085.
- Figure 4 (Eophyllophyton bellum): Open Access article Hao, S., & Xue, J. (2013). Earliest record of megaphylls and leafy structures, and their initial diversification. Chinese Science Bulletin, 58, 2784-2793.